About US
The group “Umweltanalytik und Schadstoffe (US) (Environmental Analytics and Pollutants)” focuses teaching and research on chemical and physicochemical processes related to the fate of pollutants in aquatic environments (1-10). This field divides into three major topics: A. Determination of pollutants in aquatic environments B. Transformation and disinfection processes C. Feasibility studies in cooperation with IWW Water Centre and University Duisburg-Essen
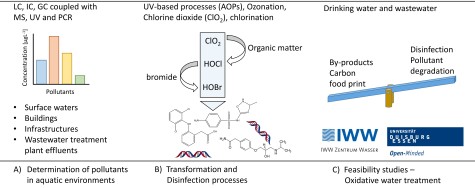
Determination of pollutants in aquatic environments
The increasing number of anthropogenic pollutants requires a continuous development of analytical tools for their determination. Pollutants can enter aquatic environments via wastewater, agriculture, or leaching from buildings and infrastructures. Especially polar compounds with low molecular mass are a challenge for analytical chemists, which can be solved by the application of new materials in separation columns. The occurrence of pollutants as well as their transformation, which may result in the formation of undesired products, are within the scope of our group using LC, IC, and GC methods coupled with online enrichment and different detectors such as MS-MS and post-column reactions.
Investigation of reaction mechanisms
A decent understanding of pollutant degradation is of utmost importance to assess if toxic products are formed. These transformation processes happen in the aquatic environment (e.g., photo-oxidation) and in technical processes, e.g., in oxidative water treatment such as ozonation, chlorination, or application of chlorine dioxide. These mechanisms are composed of a cascade of reactions until stable products are formed. The mechanisms are dependent on primary reactants and other factors such as oxygen concentration, pH, organic matter, and halogens. A case in point is the application of chlorine dioxide, which forms free chlorine in the reaction with organic matter. Free chlorine, in turn, can form free bromine in the presence of bromide, and all three oxidants (Chlorine dioxide, free chlorine, and free bromine) may be involved in pollutant transformation 8. A decent knowledge about processes, including reactions of the water matrix, is fundamental to transfer knowledge from defined laboratory scale (“pure water” experiments) to the praxis of water treatment (real waters). These complex reaction mechanisms are also involved in disinfection processes. Hardly any information is available on how oxidants react with biomolecules in pathogens such as moieties in membranes, genetic material, and proteins.
We are working on the behavior of pollutants and disinfection processes in oxidative water treatment (e.g., ozonation, photo-oxidation (e.g., UV/H2O2, UV/S2O8), chlorination and chlorine dioxide treatment). Thereby aspects of the formation of secondary oxidants, transformation product formation, and by-product formation are covered in our research activities.
Pilot studies and feasibility studies in cooperation with IWW Water Centre and University Duisburg-Essen
In cooperation with IWW Water Centre and University Duisburg-Essen, we are involved in co-supervising various projects on oxidative wastewater and drinking water treatment. These works include assessment of pollutant degradation, disinfection, formation of undesired by-products, and energy demand.
Literature