New Publication in Environmental Science & Technology
Kinetics of Aromatic Nitrogen-Containing Heterocycle Oxidation with Chlorine Dioxide and Formation of Free Available Chlorine as a Secondary Oxidant
2025/10/02
Mohammad Sajjad Abdighahroudi, Mischa Jütte, Minyi Yin, Robert Kalnins, Shaista Khaliq, Yago Batista, Torsten C. Schmidt, Holger V. Lutze
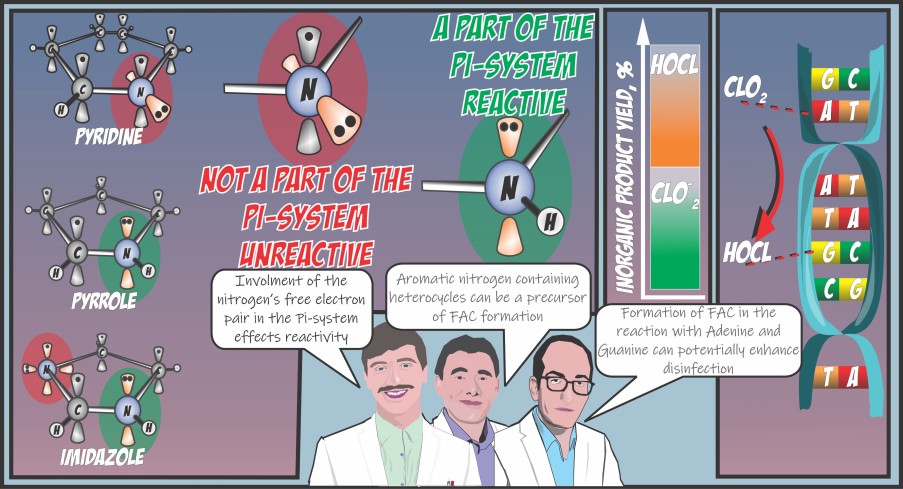
Chlorine dioxide (ClO2) is used for disinfection and preoxidation in water treatment, often as an alternative to free available chlorine (FAC) to reduce the formation of halogenated byproducts. However, the latest research has shown that FAC can be formed as a secondary oxidant in the ClO2 reaction with activated aromatic compounds, such as phenols. In this study, the reaction of ClO2 with aromatic nitrogen-containing heterocycles (NCHs) is investigated as another possible FAC precursor. Six-membered aromatic NCHs showed low reactivity (rate constants <10–3 M–1 s–1) while five-membered rings, namely pyrrole and imidazole, were more reactive. Pyrrole reacted with a rate constant of (1.18 ± 0.3) × 103 M–1 s–1 and formed 7 to 45% FAC depending on the ClO2 to pyrrole ratios. The imidazole reaction was pH-dependent since its anionic species (pKa = 14.4) rate constant was (2.08 ± 1.4) × 106 M–1 s–1. However, the reaction at pH 7 is relatively slow (6.7 × 10–2 M–1 s–1). Imidazole also showed 37% FAC formation. Nucleobases, adenine, guanine, as well as the pharmaceutical dexmedetomidine, have imidazole moieties and show a very similar reaction rate constant, FAC yield, and ClO2 consumption in their reaction with ClO2, pointing out the relevance of aromatic nitrogen-containing heterocycles in ClO2 application.
