New Publication in Water Research
Oxidation of the nitrogen-free phosphonate antiscalants HEDP and PBTC in reverse osmosis concentrates: Reaction kinetics and degradation rate
08.03.2023
Xenia A. M. Mutke, Kittitouch Tavichaiyuth, Felix Drees, Holger V. Lutze, Torsten C. Schmidt
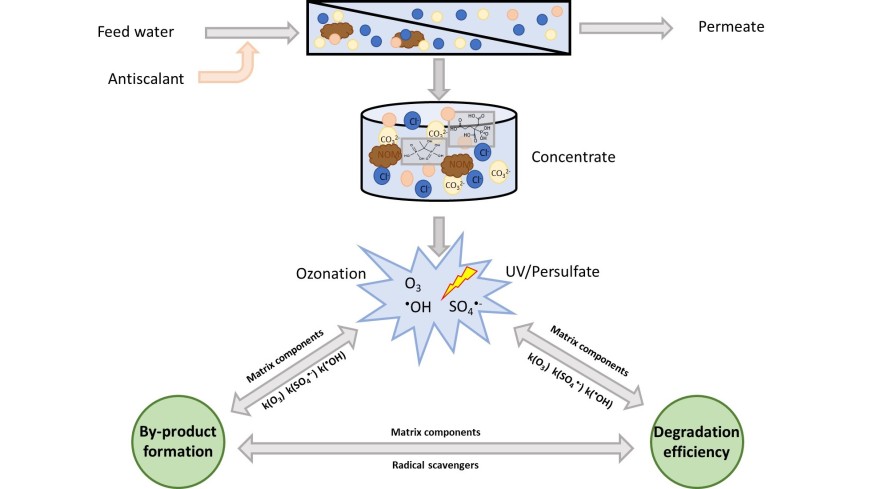
Reverse osmosis (RO) is an advanced technology used to produce potable water from a variety of water sources, including surface water, seawater and wastewater. The yield of the product water from the RO systems is increased by the addition of antiscalants which prevent scaling from calcium and other ions. Removal of antiscalants from RO concentrate can induce the precipitation of oversaturated scale-forming substances, enable additional water recovery from RO concentrates, and reduce the risk of eutrophication after concentrate disposal into the receiving water (e.g., river water). This study aims to provide a better insight into oxidation reactions of the N-free phosphonate antiscalants 1-hydroxyethane-1,1-diphosphonic acid (HEDP) and 2-phosphonobutane-1,2,4-tricarboxylic acid (PBTC) with ozone, hydroxyl radical (•OH) and sulfate radicals (SO4•−). Ozone barely reacts with HEDP and PBTC at pH 7 (k < 10 M-1 s − 1), while second order reaction rates of SO4•− and •OH were determined to be in the range 107–108 M-1 s − 1.
Sulfate, silicate and chloride matrices increased HEDP ozone degradation rate possibly due to metal complexation effect. Whereas carbonate and chloride hindered PBTC ozone degradation, and natural organic matter (NOM) inhibited both HEDP and PBTC degradation through scavenging of •OH. The SO4•−- radical based oxidation process of HEDP and PBTC is mainly inhibited by carbonate and NOM, interestingly only HEDP degradation is inhibited by chloride whereby the PBTC could not be fully degraded (degradation < 60%). The oxidation of PBTC is in real RO concentrates in both processes limited to 10% degradation, whereas HEDP could be degraded up to 60% with ozone and UV/persulfate application.
