New Publication in E&ST Water
Influences of pH, Reagent Dose, and Water Matrix Components on the Formation and Utilization of Hydroxyl Radicals in the Oxidation of Bisphenol S and para-Chlorobenzoic Acid by the Fenton Reaction
2023/02/07
Hanna Laura Wiegand, Mischa Jütte, Katharina Klein, Annika Kranefuß, Cheolyong Kim, Holger Volker Lutze, and Torsten Claus Schmidt
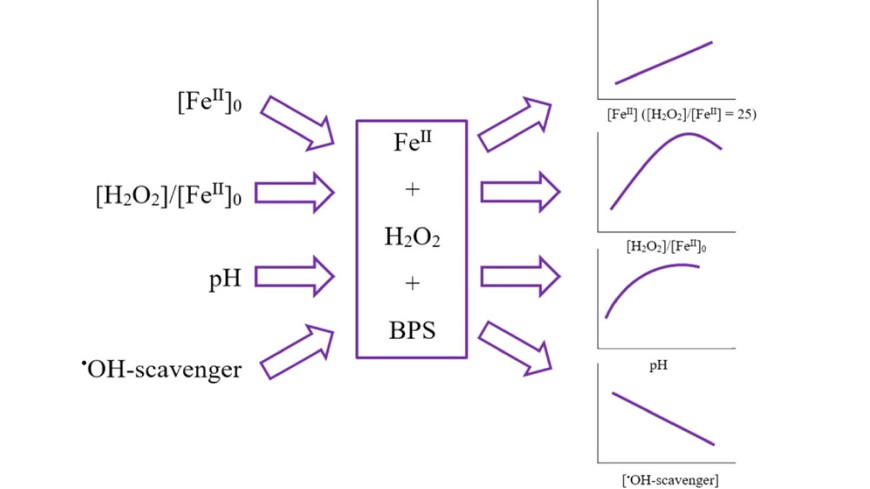
Factors influencing the efficiency of Fenton-based degradation processes are widely discussed in the literature, including the question of involved reactive species (i.e., reactive iron species such as ferryl vs hydroxyl radicals). The present study investigates the Fenton reaction in terms of degradation rates under different reaction conditions (i.e., reactant concentrations, pH, and presence of matrix constituents) using two model compounds (bisphenol S and para-chlorobenzoic acid). Fenton reaction was reported to require acidic conditions with an optimum at pH 3. However, the present study has shown that at very acidic pH (pH ≤ 2), the reduction of Fe(III) to Fe(II) is strongly hampered, lowering the efficiency of the Fenton reaction dramatically. Furthermore, the present study provides evidence that pollutants such as BPS can be degraded at pH 7 in the Fenton process, in the presence of Suwannee River NOM. This indicates that the Fenton reaction may indeed be applied for pollutant degradation at neutral pH.
