New Publication in Water Research
Linking reaction rate constants and isotope fractionation of ozonation reactions using phenols as probes
2022/01/25
Jens Terhalle, Simon E. Nikutta, Dawid L. Krzeciesa, Holger V. Lutze, Maik A. Jochmann, Torsten C.Schmidt
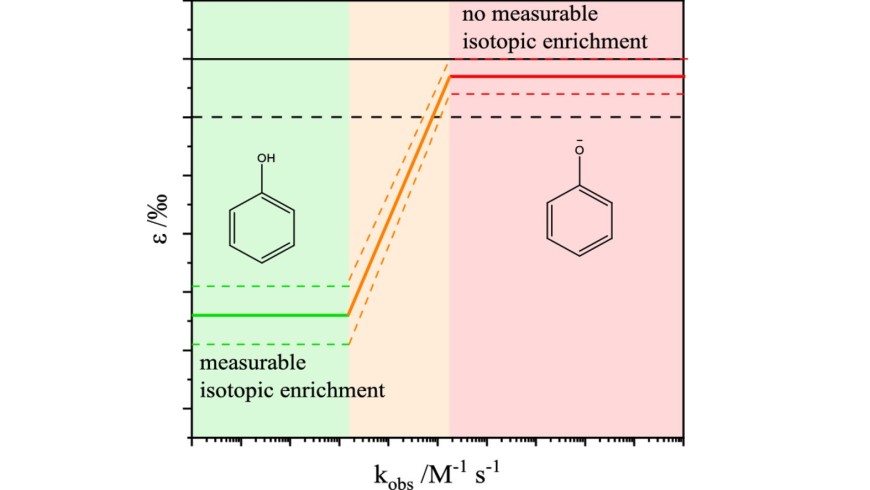
Ozonation is nowadays a widely used method in drinking water treatment for disinfection and pollutant control. However, transformation products of ozonation can be more toxic than their parent compounds. Therefore, the knowledge of the reaction mechanisms and product formation is essential for a safe application. Different analytical methods such as high-resolution mass spectrometry (HRMS) and compound-specific isotope analysis (CSIA) can be applied to elucidate products and primary attack positions of oxidation agents such as ozone. During the investigation of the ozonation of phenolic compounds in water by CSIA, a reaction rate depending carbon isotope fractionation was observed. The fractionation strongly depends on the phenol speciation. With decreasing pH values and reaction rates <105 M−1 s−1, the isotope enrichment factor ε increases (ε is between -5.2 and -1.0‰). For faster reactions (>105 M−1 s−1), the carbon isotope enrichment was not significant anymore (ε is between -1.0 and 0‰). Based on these data a concept to correlate isotope enrichment factors with kinetic data for aromatic compounds is proposed. The additional investigation of aliphatic double and triple bonds did not fit this correlation suggesting different rate-limiting steps. However, double and triple bond showed a similar enrichment factor, which implies the same rate-limiting step in the reaction with ozone, the monodentate addition of ozone.
