New Publication in CEJ
Oxidation of bromide by heat-activated persulfate – Effects of temperature and the organic matter surrogate phenol on kinetics and stoichiometry
2022/01/18
Cheolyong Kim, Torsten C Schmidt, Holger V. Lutze
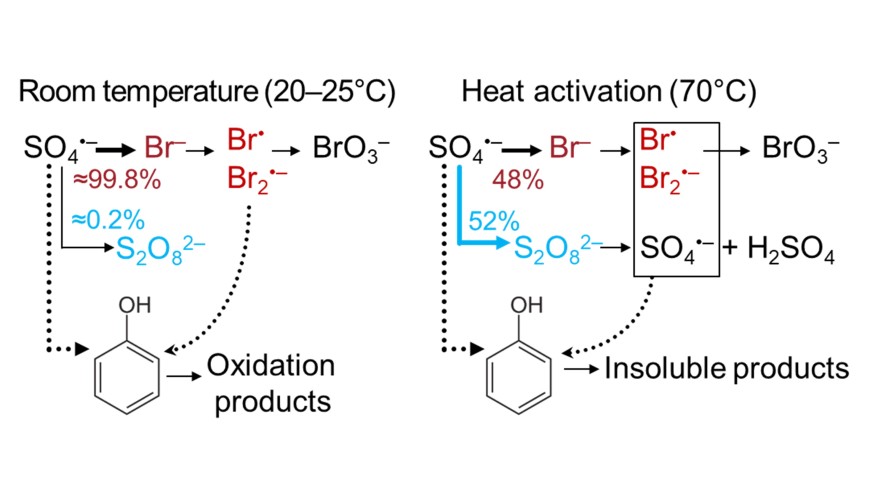
Reaction mechanisms of sulfate radical with bromide and organic substances can be largely altered at elevated temperature in heat-activated persulfate process due to different activation energies. This study investigated kinetics and stoichiometry of bromide oxidation in the heat-activated persulfate process. We postulated that reaction of sulfate radical with persulfate, which has relatively low reaction rate (6.3 × 105 M−1s−1), may become an important reaction at elevated temperatures. Persulfate decomposition was inhibited in the presence of sulfate radical scavenger phenol at low temperature (40°C) whereas phenol affected persulfate decomposition only at < 50°C but not at higher temperatures. This was because the second order reaction rate constant of sulfate radical with persulfate became higher (assumed as 1010 M−1s−1) than with phenol at a temperature > 50°C, which can be explained by a high activation energy (estimated activation energy: 311 kJ/mol). Bromate formation was inhibited in the presence of phenol. However, phenol oxidation was not affected by the presence of Br−, even though Br− reacts faster with sulfate radicals than phenol and was present in excess. This indicated that formed reactive bromine species such as Br. and Br2 .− oxidize phenol under reformation of bromide. Formation of insoluble products was also observed indicating polymerization of phenol. This can be explained by lack of dissolved oxygen under conditions of heat-activation.
